James J Ireland, PhD

Education/Degree Information
BS, 1965-69, Austin Peay State College, Clarksville, TN; PhD, 1971-75, University of Tennessee (Knoxville), Knoxville, TN; Ford Foundation Postdoctoral Fellow, 1975-77, University of Michigan, Ann Arbor, MI; Senior NIH Fellow, 1985-87, OBGYN, Yale School of Medicine, New Haven, CT
Research Interests
Research interests: Dr. Ireland's laboratory has been continuously funded since 1979 by NSF, NIH or USDA for research primarily using the bovine model to study ovarian function. I am keenly interested development of new genetic or therapeutic approaches to quantify and regulate the ovarian reserve and to improve responsiveness to gonadotropin therapy and fertility in individuals with a small ovarian reserve (total number of morphologically healthy follicles/oocytes in ovaries).
My laboratory uses the bovine model to determine if there is a link between a small ovarian reserve, fertility and health, reliability of anti-M�llerian hormone (AMH) as a biomarker for size of the ovarian reserve, fertility and health, and impact of excessive FSH doses on ovarian function, egg quality and embryo survival in individuals with a small ovarian reserve.
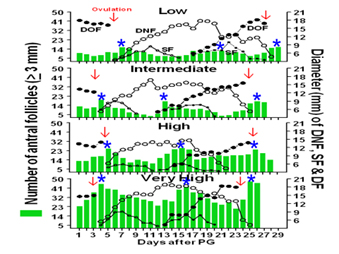
Background: The cattle in Figure 1 have two follicular waves during a 21-day estrous cycle. Open circles in Fig. 1 represents the growth pattern for the first wave dominant non-ovulatory follicle (DNF). The largest dark circles depict growth of the dominant ovulatory follicle (DF). The smaller dark circles represent the growth pattern for the largest subordinate follicles. We discovered that peak (see blue stars in Fig. 1) number of antral follicles (? 3 mm in diameter, see green bars in Fig. 1) growing during each follicular wave, hereafter referred to as antral follicle count (AFC), is very highly repeatable within individuals (0.85 to 0.95, 1 = perfect), despite the high variability among animals (depicted as Low, Intermediate, High, Very High in Fig. 1). We also discovered that the high variation in AFC is positively associated with the total number of the different types of morphologically healthy follicles and oocytes in ovaries (ovarian reserve) of cattle. For example, young adult cattle with a low AFC (?15 follicles per wave) have ~ 80% fewer follicles of all types than cattle with a higher AFC (>25 follicles)! These discoveries make the bovine model unique because individuals can be phenotyped reliably based on AFC, or anti-M�llerian hormone (AMH, a moderately heritable genetic trait highly positively correlated with AFC) to indirectly predict relative size of the ovarian reserve. Using this model, we determined that cattle with a low vs a high AFC have heightened gonadotropin secretion during follicular waves, but very low circulating concentrations of AMH, a small ovarian reserve, reduced responsiveness of the ovary to gonadotropin stimulation, reduced oocyte quality, diminished capacity to produce ovarian steroids especially progesterone (low levels of progesterone are associated with high rates of embryo mortality in cattle) and testosterone, and in some instances lower fertility and shorter herd longevity. These discoveries validated utility of the bovine model to elucidate the mechanisms whereby the inherently high variation in the ovarian reserve negatively impacts ovarian function and fertility, and whether AMH is predictive of fertility, health, and herd longevity in dairy cows, which represent two of my research goals.
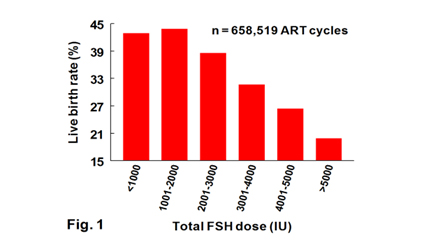 We analyzed data from SART for over 500,000 women subjected to fresh autologous ART cycles to determine if total FSH dose during ovarian stimulation was linked to live birth rates. Results (Fig.1) show a highly significant (p<0.0001) decrease in live birth rate with increasing total FSH dose. This inverse relationship remained clinically significant (P<0.0001) regardless of donor age, oocyte recovery number, length of treatment, BMI, health, and reason for IVF. This correlative study provided the rationale to test the hypothesis that excessive FSH doses are detrimental to ART outcomes. The third research goal of my research program is to use the small ovarian reserve heifer (SORH) model to determine impact of different FSH doses during superovulation on ovarian function, egg quality and embryo survival. Because a small ovarian reserve is the major contributor to infertility in women subjected to ART (diagnosis frequency= 18%), the SORH model is an appropriate biomedical model for women with a low ovarian reserve. Recently, in collaboration with Dr. Keith Latham, we established that excessive FSH doses during ovarian stimulation of the SORH model do not increase number of ovulatory-size follicles but cause follicular hyperstimulation dysgenesis (FHD) in a high proportion of ovulatory-size follicles. FHD is characterized by the simultaneous disruption of multiple signaling pathways in granulosa and cumulus cells and oocytes critical for folliculogenesis, steroidogenesis, ovulation, luteinization and cell survival which may also impair oocyte quality and hinder ART outcomes.
We analyzed data from SART for over 500,000 women subjected to fresh autologous ART cycles to determine if total FSH dose during ovarian stimulation was linked to live birth rates. Results (Fig.1) show a highly significant (p<0.0001) decrease in live birth rate with increasing total FSH dose. This inverse relationship remained clinically significant (P<0.0001) regardless of donor age, oocyte recovery number, length of treatment, BMI, health, and reason for IVF. This correlative study provided the rationale to test the hypothesis that excessive FSH doses are detrimental to ART outcomes. The third research goal of my research program is to use the small ovarian reserve heifer (SORH) model to determine impact of different FSH doses during superovulation on ovarian function, egg quality and embryo survival. Because a small ovarian reserve is the major contributor to infertility in women subjected to ART (diagnosis frequency= 18%), the SORH model is an appropriate biomedical model for women with a low ovarian reserve. Recently, in collaboration with Dr. Keith Latham, we established that excessive FSH doses during ovarian stimulation of the SORH model do not increase number of ovulatory-size follicles but cause follicular hyperstimulation dysgenesis (FHD) in a high proportion of ovulatory-size follicles. FHD is characterized by the simultaneous disruption of multiple signaling pathways in granulosa and cumulus cells and oocytes critical for folliculogenesis, steroidogenesis, ovulation, luteinization and cell survival which may also impair oocyte quality and hinder ART outcomes.
I collaborate with Dr. Keith Latham, Dr. Richard Pursley, and Dr. Rob Tempelman in the Animal Science Department at Michigan State University (http://www.ans.msu.edu/ ) to conduct our planned studies in cattle. I also collaborate with university scientists, industry representatives, and administrators in NIH and USDA-NIFA to advance use of domestic species as dual purpose comparative models to resolve critical biological problems common to both animal agriculture and biomedicine (http://www.adsbm.msu.edu/).
Key References
Burns DS, Jimenez-Krassel FJ, Ireland JLH, Knight PG, Ireland JJ. Numbers of antral follicles during follicular waves in cattle: evidence for high variation among animals, very high repeatability in individuals, and an inverse association with serum follicle-stimulating hormone concentrations. Biol Reprod 2005; 73:54-62.
Ireland JJ, Ward F, Jimenez-Krassel F, Ireland JLH, Smith GW, Lonergan P, Evans ACO. Follicle numbers are highly repeatable within individual animals but are inversely correlated with FSH concentrations and the proportion of good-quality embryos after ovarian stimulation in cattle. Hum Reprod 2007; 22:1687-1695.
Ireland JJ, Roberts RM, Palmer GH, Bauman DE, Bazer FW. A commentary on domestic animals as dual-purpose models that benefit agricultural and biomedical research. J Anim Sci 2008; 86:2797-2805.
Roberts RM, Smith GW, Bazer FW, Cibelli J, Seidel GE, Jr., Bauman DE, Reynolds LP, Ireland JJ. Farm animal research in crisis. Science 2009; 324:468-469.
Ireland JJ, Smith GW, Scheetz D, Jimenez F, Folger JK, Ireland JLH, Mossa F, Lonergan P, Evans ACO. Does size matter in females? An overview of the impact of the high variation in the ovarian reserve on ovarian function and fertility, utility of ant-Mullerian hormone as a diagnostic marker for fertility and causes of variation in the ovarian reserve in cattle. Reprod Fertil Dev 2011; 23:1-14.
Baker VL, Brown MB, Luke B, Smith GW, Ireland JJ. 2015. Gonadotropin dose is negatively correlated with live birth rate: analysis of more than 650,000 assisted reproductive technology cycles. Fertil Steril 104: 1145-1152.
Mossa F, Jimenez F, Ireland JJ. 2017. Anti-M?llerian Hormone (AMH) and fertility management in agricultural species. Reproduction. 154: R1-R11.
Jimenez F, Scheetz D., Neuder L, Pursley J, Ireland JJ. 2017. A single ultrasound determination of ?25 follicles ?3 mm in diameter in dairy heifers is predictive of a reduced productive herd life. J Dairy Sci. 100:5019-5027.
Jimenez F, Ireland JLH, Kronemeyer C, Wilson-Alvarado A, Ireland JJ. 2018. Development of the �waveless� bovine model. Anim Reprod Sci. 195:80-88.
Nawaz MY, Jimenez-Krassel F, Steibel JP, Lu Y, Baktula A, Vukasinovic N, DeNise SK, Neuder L, Ireland JLH, Ireland JJ, Tempelman RJ. 2018. Genomic heritability and genome wide association study of anti-M�llerian hormone in Holstein heifers. J Dairy Sci. 101:8063-8075.
Clark Z, Thakur M, Leach RE, Ireland JJ. 2021. FSH dose is negatively correlated with oocyte retrieval: analysis of ~650,000 ART cycles that previously identified an inverse relationship between FSH dose and live birth rate. J Assist Reprod Genet 38:1787-1797.
Spencer T, Wells KD, Lee K, Telugu BP, Bartol FF, Blomberg L, Schook LB, Dawson H, Lunney JK, Driver JP, Davis TA, Donovan SM, Dilger RN, Saif LJ, Moeser A, McGill JL, Smith GWS, Ireland JJ. 2022. Future of biomedical, agricultural, and biological research systems using domesticated animals. Biol Reprod 106:629-638.
Karl KR, Clark ZL, Tempelman RJ, Latham KE, Ireland JJ. 2022. Limitations in use of ovarian biomarkers to predict the superovulation response in small ovarian reserve heifers. Theriogenology 182:53-62.
Clark ZL, Karl KR, Ruebel ML, Latham KE, Ireland, JJ. 2022. Excessive FSH during ovarian stimulation of cattle may induce premature luteinization of most ovulatory-size follicles. Biol Reprod 106:968-978.
Clark Z, Ruebel M, Schall P, Karl KR, Ireland JJ, Latham KE. 2022. Follicular hyperstimulation dysgenesis: a new explanation for adverse effects of excessive FSH doses during ovarian stimulation. Endocrinology 163: https://doi.org/10.1210/endocr/bqac100
Grant awards for most recent 5 years:
USDA-NIFA-AFRI & NICHD: PD, 2017-2023, Impact of Antral Follicle Count and FSH Dosage on ART Outcomes in Cattle, Dual Purpose with Dual Benefit: Research in Biomedicine and Agriculture Using Agriculturally Important Domestic Species (RO1), PAR-13-204.




 Print
Print Email
Email