A quorum sensing-system regulates virulence in the fungal pathogen Cryptococcus neoformans
Quorum sensing (QS) is a mechanism of cell-cell communication, in which secreted signaling molecules influence population function and gene expression.
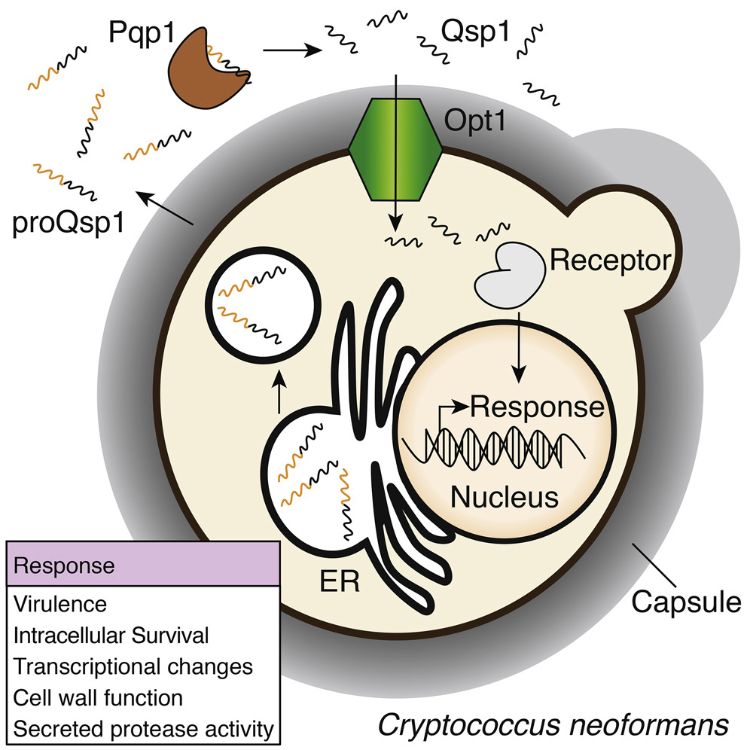
Quorum sensing (QS) is a mechanism of cell-cell communication, in which secreted signaling molecules influence population function and gene expression. Bacterial QS systems and its role in virulence has been extensively researched with especial focus on pathogenic bacteria. However, QS-like phenomena in eukaryotes, have been just reported and their functions, molecules and mechanisms remain largely unknown. QS in bacteria involve the production, detection, and response to extracellular signaling molecules called autoinducers, which are either peptides or small molecules such as acyl-homoserine lactones (AHLs). Homer et al. identified a quorum-sensing system in the pathogenic fungus Cryptococcus neoformans. The Qsp1 (quorum sensing–like peptide 1), was identified as the central signaling molecule. Qsp1 is a 11–amino acid peptide functioning intracellularly and required for fungal virulence and to control intracellular growth, cell wall, and protease activities.
Qsp1 is produced extracellularly by proteolytic cleavage of its precursor, proQsp1, which is a secreted 24–amino acid pro-peptide encoded by the QSP1 gene. CHIP-seq studies revealed that QSP1 is a direct target of three transcription factors required for virulence. To assess the role of QSP1 in virulence, mutant C. neoformas lacking QSP1 (qsp1Δ cells) were used to conduct intranasal infection of mice. C. neoformas qsp1Δ mutants exhibit attenuated infection and slowed tissue accumulation, and elicited an altered inflammatory response compared with wild-type C. neoformas. Cultivated qsp1Δ cells at room temperature form dry, wrinkled colonies, in contrast to smooth, mucoid colonies formed by wild-type cells. Mutant qsp1Δ cells treated with synthetic Qsp1 or growing near wild type cells reverted the phenotype to smooth cells, suggesting that Qsp1 promotes cell wall function at high cell densities. In addition, RNA-seq experiments revealed that many of the genes differentially expressed between mutants and wild-type cells were involved in cell wall biosynthesis.
Homer et al. elucidate additional components of the Qsp1 signaling pathway. They identified Pqp1 as the secreted protease that cleaves proQsp1 and the oligopeptide Opt1 as the responsible for transport Qsp1 to the interior of the cells. Intracellular function of Qsp1 was confirmed by expression of mature Qsp1 intracellularly, which complemented qsp1Δ phenotypes. A receptor for Qsp1 has not been recognized; though, the transcription factor Liv3 was required for most of the Qsp1 functions, and liv3Δ mutants formed dry colonies. This study demonstrates a quorum sensing system in eukaryotes, which according to Homer et al., based on the presence of some components in the ancestor of C. neoformans, is likely to have evolved prior to the emergence of the pathogenic Cryptococcus neoformans/ Cryptococcus gattii species complex.
Source:
- Homer CM, Summers DK, Goranov AI, Clarke SC, Wiesner DL, Diedrich JK, Moresco JJ, Toffaletti D, Upadhya R, Caradonna I, et al (2016) Intracellular Action of a Secreted Peptide Required for Fungal Virulence. Cell Host Microbe 19: 849–864



 Print
Print Email
Email



